The perfect saturated steam sterilisation
GMP and GLP solutions for processing heat-resistant and moisture-stable materials.
GMP
Our equipment is designed according to good manufacturing practices for pharmaceutical and biotechnology-sector applications.
The Good Manufacturing Practice GMPefer to medicinal products for human use, and define the requirements that must be met during the development, production and testing phases. In order for a medicine to be deemed safe and effective, the GMP-dictated guidelines must be observed. A fundamental requirement that guarantees pharmaceutical quality. Only by strictly adhering to the GMPs during all the production process phases and the inevitable analysis that follows, can the subsequently produced medicines be deemed effective and safe. More specifically, GMP washing equipment therefore prevents potential contamination during the various processes and keeps the different batches or products disinfected.

GMP
Our equipment is designed according to good manufacturing practices for pharmaceutical and biotechnology-sector applications.
The Good Manufacturing Practice GMPefer to medicinal products for human use, and define the requirements that must be met during the development, production and testing phases. In order for a medicine to be deemed safe and effective, the GMP-dictated guidelines must be observed. A fundamental requirement that guarantees pharmaceutical quality. Only by strictly adhering to the GMPs during all the production process phases and the inevitable analysis that follows, can the subsequently produced medicines be deemed effective and safe. More specifically, GMP washing equipment therefore prevents potential contamination during the various processes and keeps the different batches or products disinfected.

GMP
Our equipment is designed according to good manufacturing practices for pharmaceutical and biotechnology-sector applications.
The Good Manufacturing Practice GMPefer to medicinal products for human use, and define the requirements that must be met during the development, production and testing phases. In order for a medicine to be deemed safe and effective, the GMP-dictated guidelines must be observed. A fundamental requirement that guarantees pharmaceutical quality. Only by strictly adhering to the GMPs during all the production process phases and the inevitable analysis that follows, can the subsequently produced medicines be deemed effective and safe. More specifically, GMP washing equipment therefore prevents potential contamination during the various processes and keeps the different batches or products disinfected.
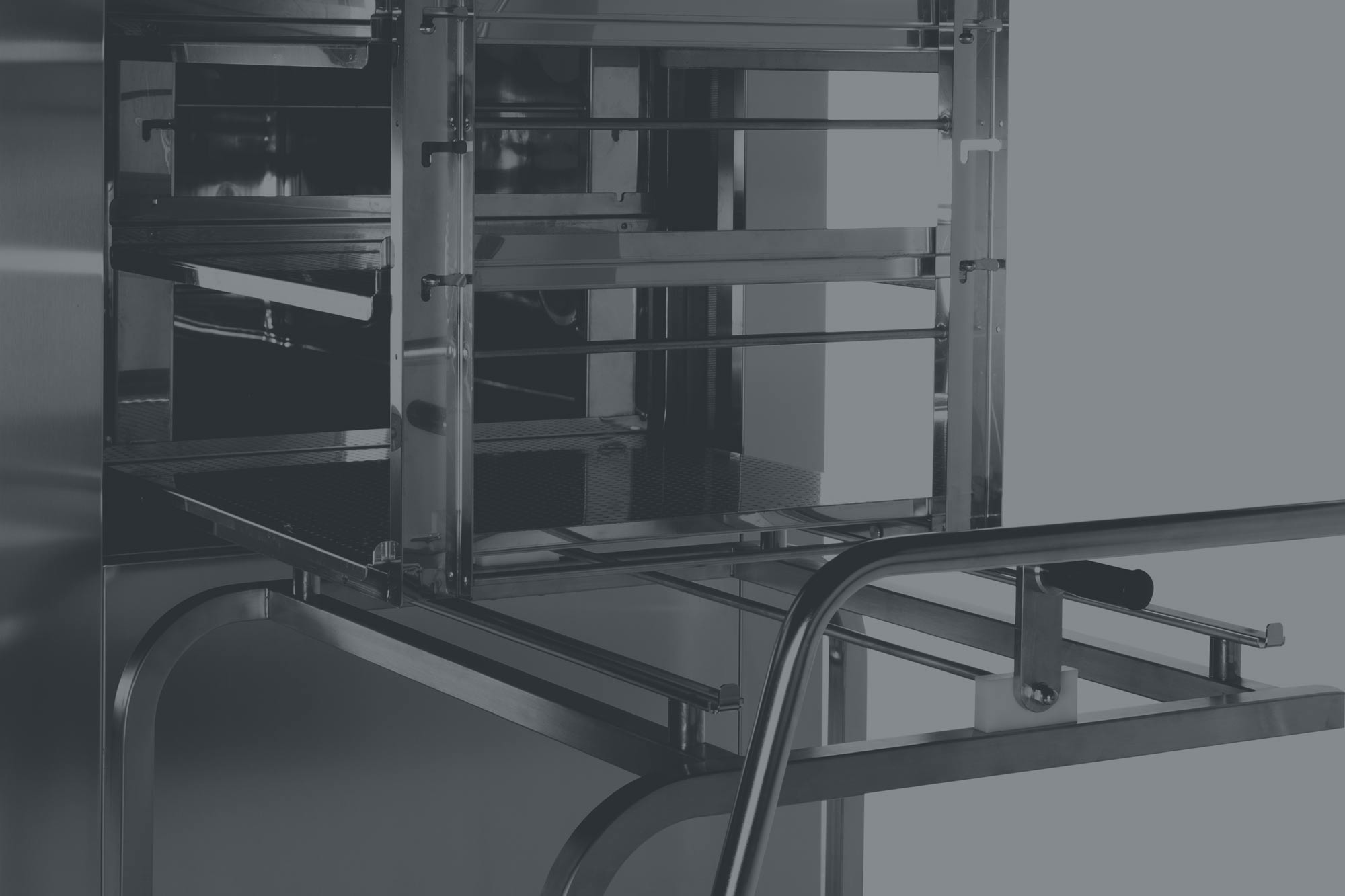
GLP
We also design good manufacturing practice-compliant equipment for research or microbiology laboratory applications.
The Good Laboratory Practices (GLP) define the organisational procedures and principals by which laboratory research for non-clinical trials is planned, conducted, controlled, recorded and reported. The GLP principles have been designed to promote the quality and validity of experimental data generated and used to determine the safety of substances contained in pharmaceuticals, pesticides, cosmetic products, veterinary medicines, food additives, feed additives and industrial chemicals, unauthorised for humans. The quality level of such data must be compared with those of other countries in order to make them mutually acceptable in all countries involved. Before GLP compliance certifications are issued, it is usually subjected to periodic checks and inspections.
GLP
We also design good manufacturing practice-compliant equipment for research or microbiology laboratory applications.
The Good Laboratory Practices (GLP) define the organisational procedures and principals by which laboratory research for non-clinical trials is planned, conducted, controlled, recorded and reported. The GLP principles have been designed to promote the quality and validity of experimental data generated and used to determine the safety of substances contained in pharmaceuticals, pesticides, cosmetic products, veterinary medicines, food additives, feed additives and industrial chemicals, unauthorised for humans. The quality level of such data must be compared with those of other countries in order to make them mutually acceptable in all countries involved. Before GLP compliance certifications are issued, it is usually subjected to periodic checks and inspections.

GLP
We also design good manufacturing practice-compliant equipment for research or microbiology laboratory applications.
The Good Laboratory Practices (GLP) define the organisational procedures and principals by which laboratory research for non-clinical trials is planned, conducted, controlled, recorded and reported. The GLP principles have been designed to promote the quality and validity of experimental data generated and used to determine the safety of substances contained in pharmaceuticals, pesticides, cosmetic products, veterinary medicines, food additives, feed additives and industrial chemicals, unauthorised for humans. The quality level of such data must be compared with those of other countries in order to make them mutually acceptable in all countries involved. Before GLP compliance certifications are issued, it is usually subjected to periodic checks and inspections.
Customisation is quick and easy
Keep up to date with the latest news from the Last Technology worldand let us steer you towards the most suitable service for you!
