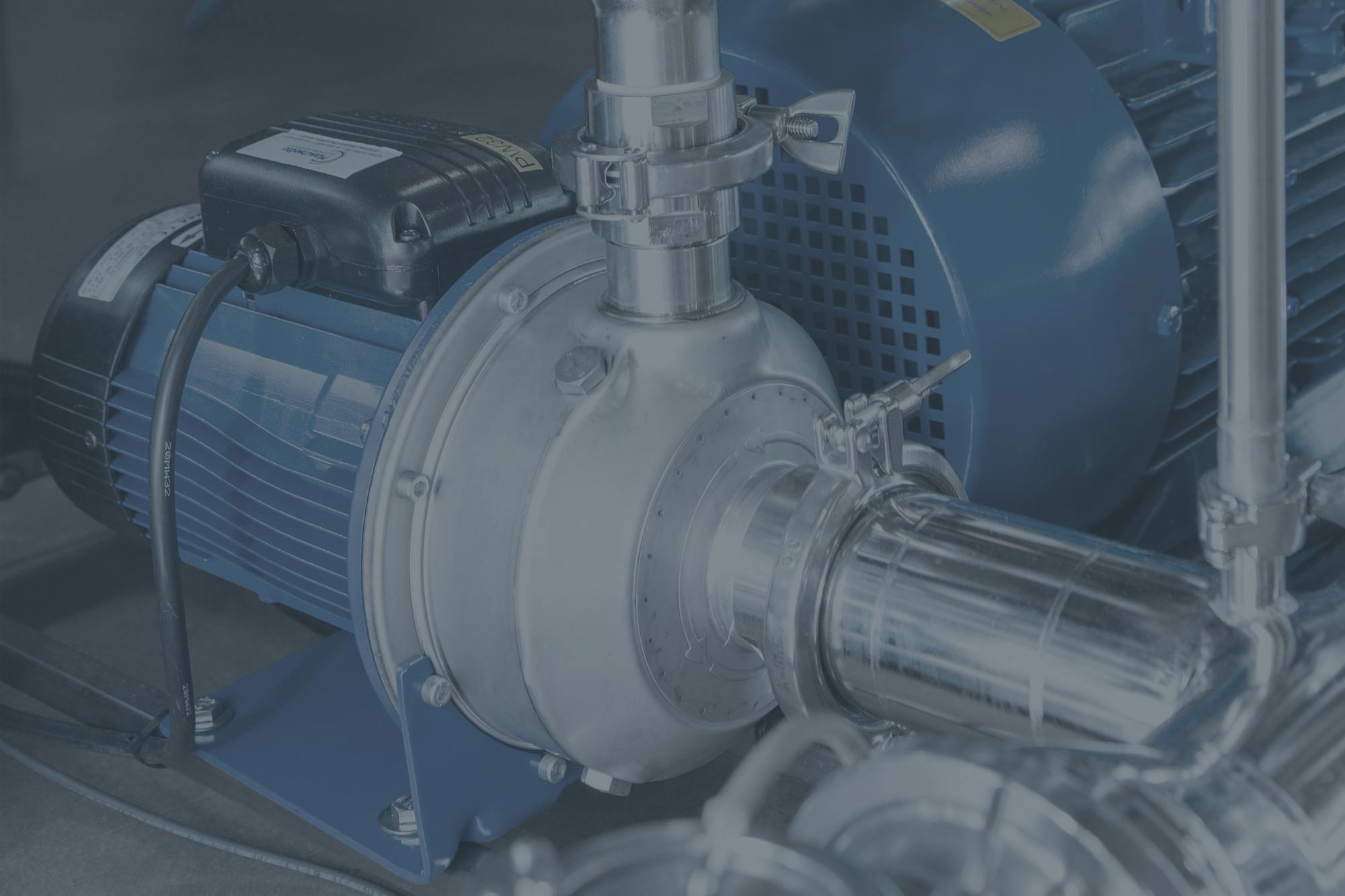
“Standard” and custom programmes for every need. he equipment process is developed by our Automation Department, according to the current codes/standards and the type of product being processed. Products go from dirty to ready to be sterilised, by means of a pre-wash, chemical and/or clean steam-added wash, rinse with PW or WFI (recirculation or direct flow) and a final HEPA 13-filtered hot air drying phase. Whilst also keeping the water TOC level, conductivity and pH level under control. During the product wash phase, chemicals and/or clean steam can be injected by means of a precise and reliable system, in order to improve the equipment’s cleaning action.
- Single-walled square or rectangular chamber in 316L or 316Ti stainless steel.
- Innovative central pumping/draining sump in 316L or 316Ti stainless steel.
- Piping and air ducts made entirely from 316L stainless steel with sanitary fittings (tri-clamp ferrules and hygienic flanges).
- 3D (dead leg)-compliant along the entire piping system and a minimum inclination of 3° of the same towards the floor drain.
- Any surfaces that come into contact with the product are mechanically polished to a roughness level of less than 0.35 micron.
- Rounded internal chamber corners to ensure a perfect clean.
- Manual folding chamber doors or vertical/horizontal automatic sliding doors (316L or 316Ti stainless steel door with a HST tempered glass inspection window).
- Chamber door oil seal made of silicone rubber.
- 316L/316Ti stainless steel components and tools, in addition to elastomers manufactured in compliance with FDA 21 CFR, Part 177.
- The chamber, heads, pipes, components and instruments are properly insulated by a cutting-edge material.
- Zone separation by means of a 304 or 316L/316Ti stainless steel “bio-seal” septum.
- A brand-new design that connects the utilities to the internal trolley, complete with an automatic locking and unlocking system (100% seal guaranteed)
- Manual or fully automated ergonomic product loadingand unloading solutions.
- Floor or elevated loading solutions.
cGMP washing equipment
 PHARMA DIVISION
PHARMA DIVISION
cGMP Washing Equipment Description
The UCW equipment is an industrial parts washing machine specifically designed to process wash, disinfect and dry instruments, glassware, filling machine components and large containers (IBC), etc.
![]() SOLIDS AND SEMI-SOLIDS
SOLIDS AND SEMI-SOLIDS
![]() WATER + CLEANERS or SOLVENTS + AIR
WATER + CLEANERS or SOLVENTS + AIR
![]() 20°C – 120°C
20°C – 120°C

“Standard” and custom programmes for every need. he equipment process is developed by our Automation Department, according to the current codes/standards and the type of product being processed. Products go from dirty to ready to be sterilised, by means of a pre-wash, chemical and/or clean steam-added wash, rinse with PW or WFI (recirculation or direct flow) and a final HEPA 13-filtered hot air drying phase. Whilst also keeping the water TOC level, conductivity and pH level under control. During the product wash phase, chemicals and/or clean steam can be injected by means of a precise and reliable system, in order to improve the equipment’s cleaning action.
- Single-walled square or rectangular chamber in 316L or 316Ti stainless steel.
- Innovative central pumping/draining sump in 316L or 316Ti stainless steel.
- Piping and air ducts made entirely from 316L stainless steel with sanitary fittings (tri-clamp ferrules and hygienic flanges).
- 3D (dead leg)-compliant along the entire piping system and a minimum inclination of 3° of the same towards the floor drain.
- Any surfaces that come into contact with the product are mechanically polished to a roughness level of less than 0.35 micron.
- Rounded internal chamber corners to ensure a perfect clean.
- Manual folding chamber doors or vertical/horizontal automatic sliding doors (316L or 316Ti stainless steel door with a HST tempered glass inspection window).
- Chamber door oil seal made of silicone rubber.
- 316L/316Ti stainless steel components and tools, in addition to elastomers manufactured in compliance with FDA 21 CFR, Part 177.
- The chamber, heads, pipes, components and instruments are properly insulated by a cutting-edge material.
- Zone separation by means of a 304 or 316L/316Ti stainless steel “bio-seal” septum.
- A brand-new design that connects the utilities to the internal trolley, complete with an automatic locking and unlocking system (100% seal guaranteed)
- Manual or fully automated ergonomic product loadingand unloading solutions.
- Floor or elevated loading solutions.
cGMP washing equipment
 PHARMA DIVISION
PHARMA DIVISION
cGMP Washing Equipment Description
The UCW equipment has been specifically designed to process wash, disinfect and dry instruments, glassware, filling machine components and large containers (IBC), etc.
![]() SOLIDS AND SEMI-SOLIDS
SOLIDS AND SEMI-SOLIDS
![]() WATER + CLEANERS or SOLVENTS + AIR
WATER + CLEANERS or SOLVENTS + AIR
![]() 20°C – 120°C
20°C – 120°C

cGMP washing equipment
 PHARMA DIVISION
PHARMA DIVISION
cGMP Washing Equipment Description
The UCW equipment has been specifically designed to process wash, disinfect and dry instruments, glassware, filling machine components and large containers (IBC), etc.
![]() SOLIDS AND SEMI-SOLIDS
SOLIDS AND SEMI-SOLIDS
![]() WATER + CLEANERS or SOLVENTS + AIR
WATER + CLEANERS or SOLVENTS + AIR
![]() 20°C – 120°C
20°C – 120°C
“Standard” and custom programmes for every need. he equipment process is developed by our Automation Department, according to the current codes/standards and the type of product being processed. Products go from dirty to ready to be sterilised, by means of a pre-wash, chemical and/or clean steam-added wash, rinse with PW or WFI (recirculation or direct flow) and a final HEPA 13-filtered hot air drying phase. Whilst also keeping the water TOC level, conductivity and pH level under control. During the product wash phase, chemicals and/or clean steam can be injected by means of a precise and reliable system, in order to improve the equipment’s cleaning action.
- Single-walled square or rectangular chamber in 316L or 316Ti stainless steel.
- Innovative central pumping/draining sump in 316L or 316Ti stainless steel.
- Piping and air ducts made entirely from 316L stainless steel with sanitary fittings (tri-clamp ferrules and hygienic flanges).
- 3D (dead leg)-compliant along the entire piping system and a minimum inclination of 3° of the same towards the floor drain.
- Any surfaces that come into contact with the product are mechanically polished to a roughness level of less than 0.35 micron.
- Rounded internal chamber corners to ensure a perfect clean.
- Manual folding chamber doors or vertical/horizontal automatic sliding doors (316L or 316Ti stainless steel door with a HST tempered glass inspection window).
- Chamber door oil seal made of silicone rubber.
- 316L/316Ti stainless steel components and tools, in addition to elastomers manufactured in compliance with FDA 21 CFR, Part 177.
- The chamber, heads, pipes, components and instruments are properly insulated by a cutting-edge material.
- Zone separation by means of a 304 or 316L/316Ti stainless steel “bio-seal” septum.
- A brand-new design that connects the utilities to the internal trolley, complete with an automatic locking and unlocking system (100% seal guaranteed)
- Manual or fully automated ergonomic product loadingand unloading solutions.
- Floor or elevated loading solutions.
We may be small, but we can achieve great things.
Thanks to our customisable after-sales service packages, we offer solutions to suit every need. Choose the one that suits you best!
Customisation is quick and easy
Keep up to date with the latest news from the Last Technology worldand let us steer you towards the most suitable service for you!